Kenttämaa Labs
Analytical & Physical Organic Chemistry
Research on aviation fuels
![]()
The Kenttämaa group engages in research focused on the chemical characterization of jet, diesel, and rocket fuels, which involves using state-of-the-art analytical chemistry instrumentation, such as two-dimensional gas chromatography with flame ionization detection (GC×GC/FID) and mass spectrometry detection (GC×GC/MS). Previous research efforts have included investigating the composition, physical properties, and combustion properties of jet fuels and jet fuel surrogate mixtures,1 as well as how the different aromatic hydrocarbons present in conventional and alternative jet fuels contribute to the swelling and physical properties of O-ring seals used in aircraft fuel systems.2 Other research efforts have investigated how changes in the chemical composition of jet fuel influence an array of physical properties, including the development of various correlation algorithms for predicting the density of jet fuel based on its hydrocarbon composition measured with GC×GC/FID.3 Further, the influence of common laboratory practices, such as sample storage, handling, preparation, and analysis, on the precision of flash point and GC×GC/FID measurements has been examined. Current research is focused on developing methods for quantifying alkylphenols and other oxygenates in jet fuel by using two-dimensional gas chromatography coupled with mass spectrometry (GC×GC/MS) and chemical ionization. This is important as oxygenates have been found to shorten the storage time of aviation fuels. Further, long-term studies are carried out to investigate how storage duration and container type influence the ability to detect high-molecular weight contaminants in jet fuel.
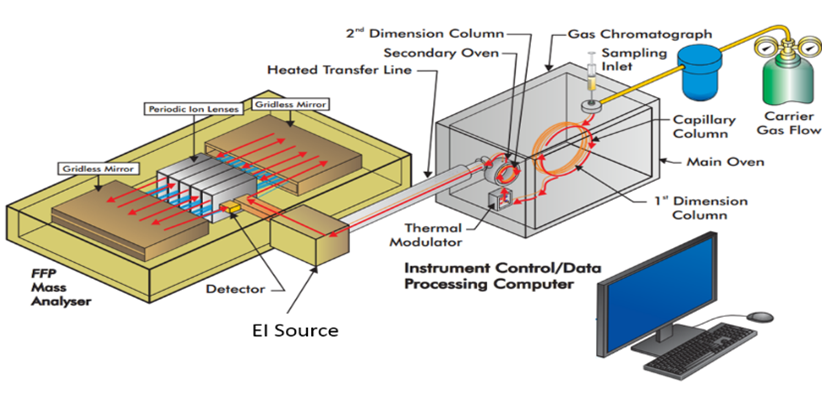
Figure 25. Diagram of the GC×GC/time-of-flight mass spectrometer.
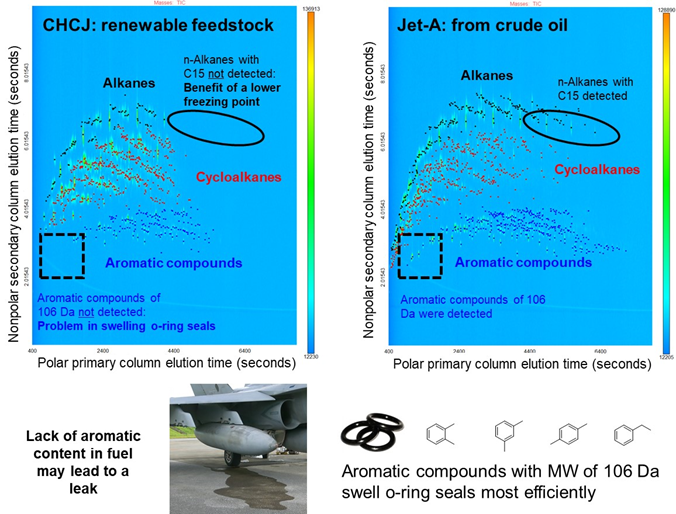
Figure 26. Total ion chromatograms obtained using the GC×GC/time-of-flight mass spectrometer for two fuels. The lack of small aromatic compounds from CHCJ fuel means that the o-ring seals of the fuel circulation system will not swell enough to prevent fuel leaks.
References:
- Luning Prak, D.; Romanczyk, M.; Wehde, K.; Ye, S.; McLaughlin, M.; Luning Prak, P.; Foley, M.; Kenttämaa, H.; Trulove, P.; Kilaz, G.; Xu, L.; Cowart, J. Analysis of Catalytic Hydrothermal Conversion Jet Fuel and Surrogate Mixture Formulation: Components, Properties, and Combustion. Energy Fuels 2017, 31, 13802-13814.
- Romanczyk, M.; Ramirez Velasco, J.; Xu, L.; Vozka, P.; Dissanayake, P.; Wehde, K.; Roe, N.; Keating, E.; Kilaz, G.; Trice, R.; Luning Prak, D.; Kenttämaa, H. The Capability of Organic Compounds to Swell Acrylonitrile Butadiene O-rings and Their Effects on O-ring Mechanical Properties. Fuel 2019, 238, 483-492.
- Vozka, P.; Modereger, B.; Park, A.; Zhang, W.T.J.; Trice, R.; Kenttämaa, H.; Kilaz, G. Jet Fuel Density Via GC×GC-FID. Fuel 2019, 235, 1052-1060.